
289
The easiest and most pragmatic approach was to decrease the level of sulphur in steel
as much as possible. To understand this approach, it is necessary to understand the origin
of sulphur in steel. The dominant origin of sulphur in steel is from coke. Coal used for
coke production can have up to 1% of S! Large amount of sulphur that is charged into
blast furnace will be dissolved in pig iron. The most effective way to decrease sulphur
content in steel is to add large amounts of Ca (as lime powder) during ladle treatment of
both pig iron before steel production and ladle treatment of steel. The biggest obstacles
for this activity are very large costs and sometime very high investment in new
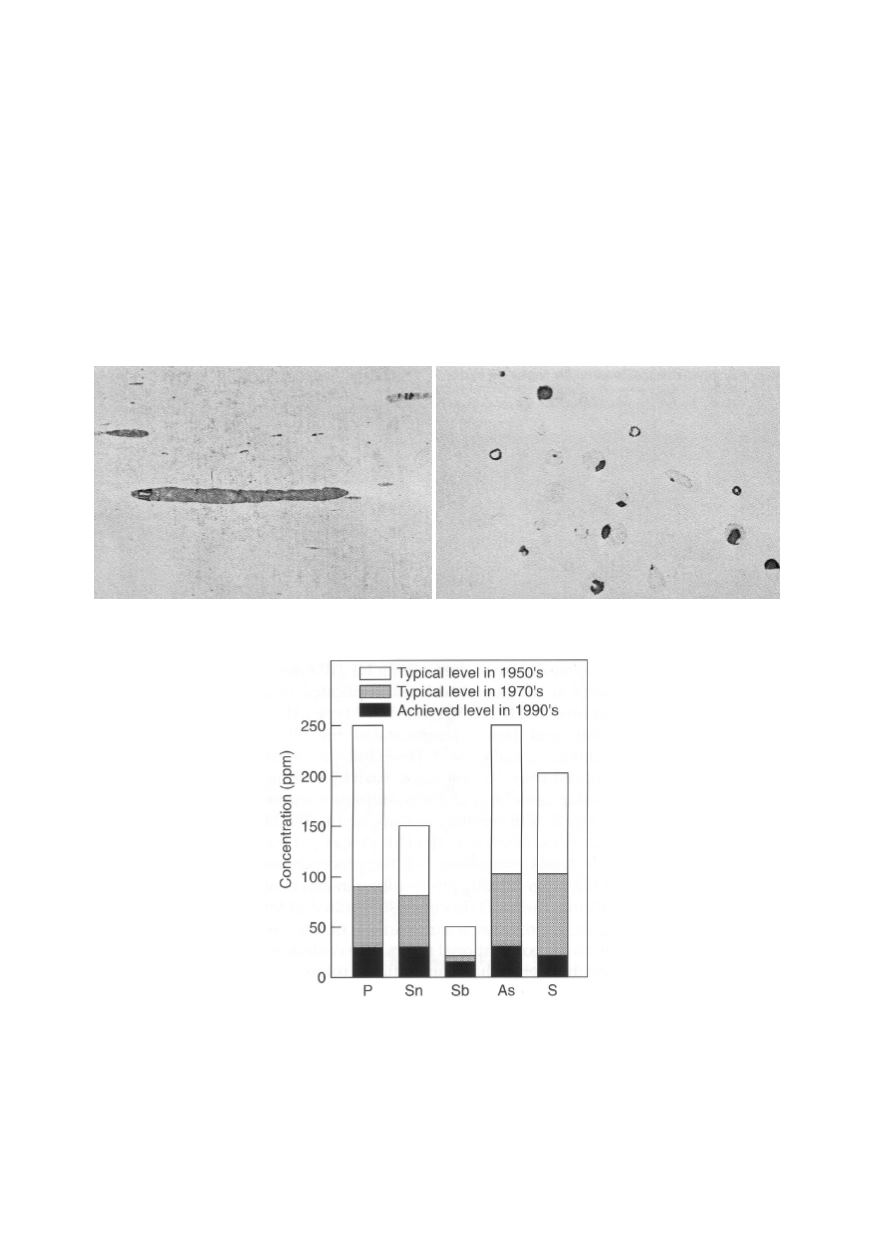
equipment. Figure 6 shows the change of sulphur and other impurities in steel for the
period 1950-2000 /8/. The only problem to this approach was the extremely high cost for
production of steel for mass applications. Therefore, in some respect, the whole approach
had to be changed.
Figure 5: MnS inclusions with voids in low carbon steel: elongated MnS inclusion after hot rolling
(left); refined and dispersed MnS inclusions after forging (right) /4/
Figure 6: Trends in changing of impurity levels in Cr-Mo-V rotor steels /8/
One of the advantages was the knowledge from fracture mechanics, i.e. the application
of a component with present crack in it. Therefore, the main goal was not to remove all
sulphur from steel, but to make it non effective, i.e. to eliminate sulphides as critical
particles. The solution was additional alloying with Ca or rare earth elements (RE).


















