
288
(close to 900ºC), leading to melting on the grain boundaries followed by fracture. To
avoid presence of sulphur in solid solution, the alloying with manganese was introduced.
This approach was based on high affinity between manganese and sulphur, resulting in
formation of manganese sulphide (MnS). MnS is a particle formed at temperatures close
to solidification temperature and can be present both on grain boundaries and within the
grains. In this way, the problems related to occurrence of low melting eutectic mixtures
were solved. On the other hand, presence of particles had triggered new problems. MnS
particles can be discussed in terms of stress concentration and influence on fracture
toughness. Its influence on fracture toughness depends on overall presence of sulphur and
the size and shape distribution of particles. Its surface is composed of large number of
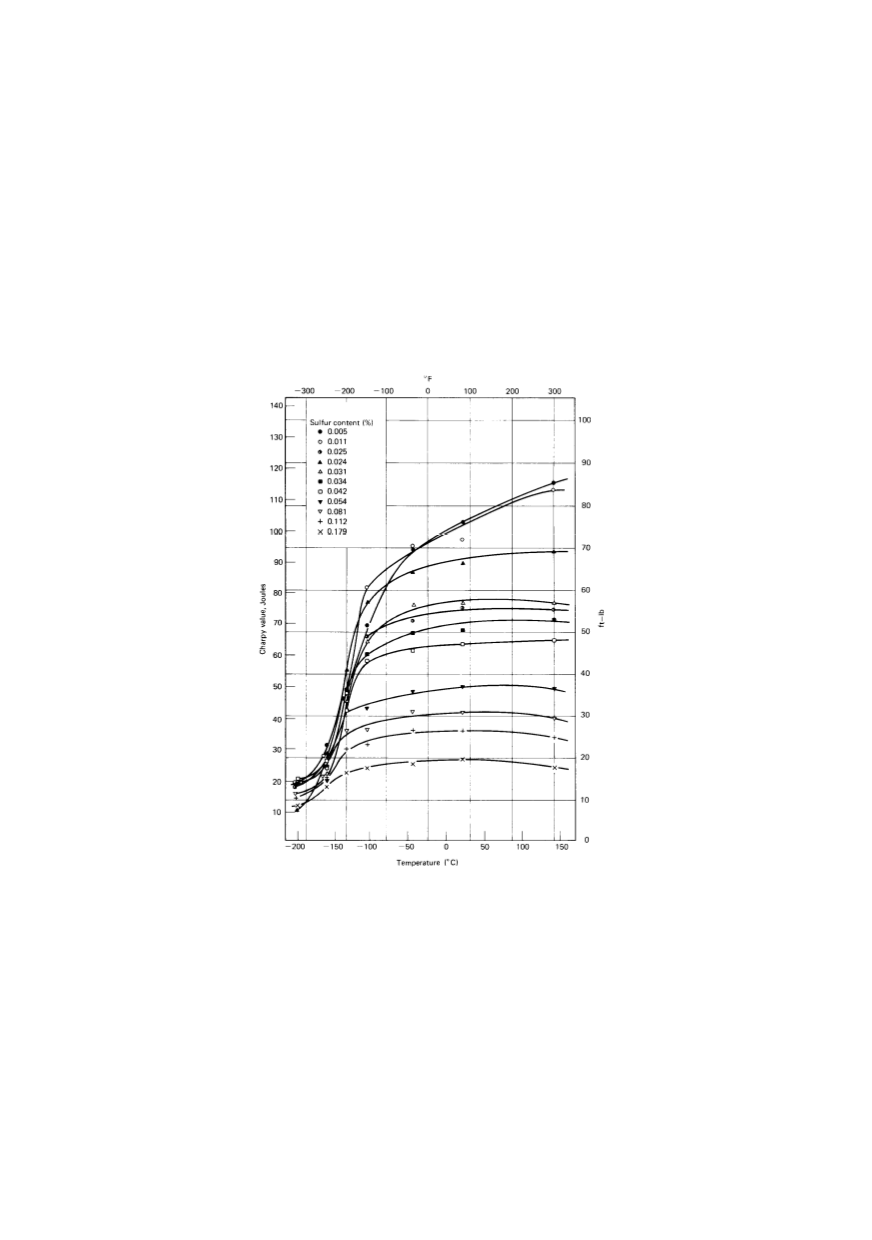
different planes, and basically MnS’s influence depends solely on its size. Figure 4 shows
the influence of the sulphur content on the toughness /7/.
Figure 4: Influence of sulphur content on transition temperature in steels /7/
3. CONTROL OF MnS INCLUSIONS
This role of Mn ensured significant rise in toughness in low carbon steels, with exce-
ptions of some medium carbon quenched and tempered steels. During further metal wor-
king, MnS inclusions can become elongated (rolling) or fractured and dispersed (forging).
Presence of long elongated MnS inclusions in as rolled steels is very dangerous, since the
edges behave as stress concentrators, leading to fracture or to lamellar tearing in welded
constructions. Also, particles themselves can be fractured and can initiate the crack that
can propagate through steel matrix and lead to fracture /4/. Figure 5 represents the
elongated MnS inclusion after hot rolling in low carbon steel. The inclusion is placed
within the ferrite microstructure.


















