
287
premium yield and cause serious problems in all steps in the production (deformation,
machining, heat treatment and welding). Since their removal is mandatory during
treatment of molten steel, they will not be discussed.
The main focus is related to the formation of non-metallic inclusions during cooling in
solid state. Sulphides, carbides, and nitrides precipitate under normal conditions during
cooling of steel below solidus temperatures.
The shape and chemical composition of inclusions can be very different by its shape.
They can be formed of one or more chemical compounds, or can be spherically, cylin-
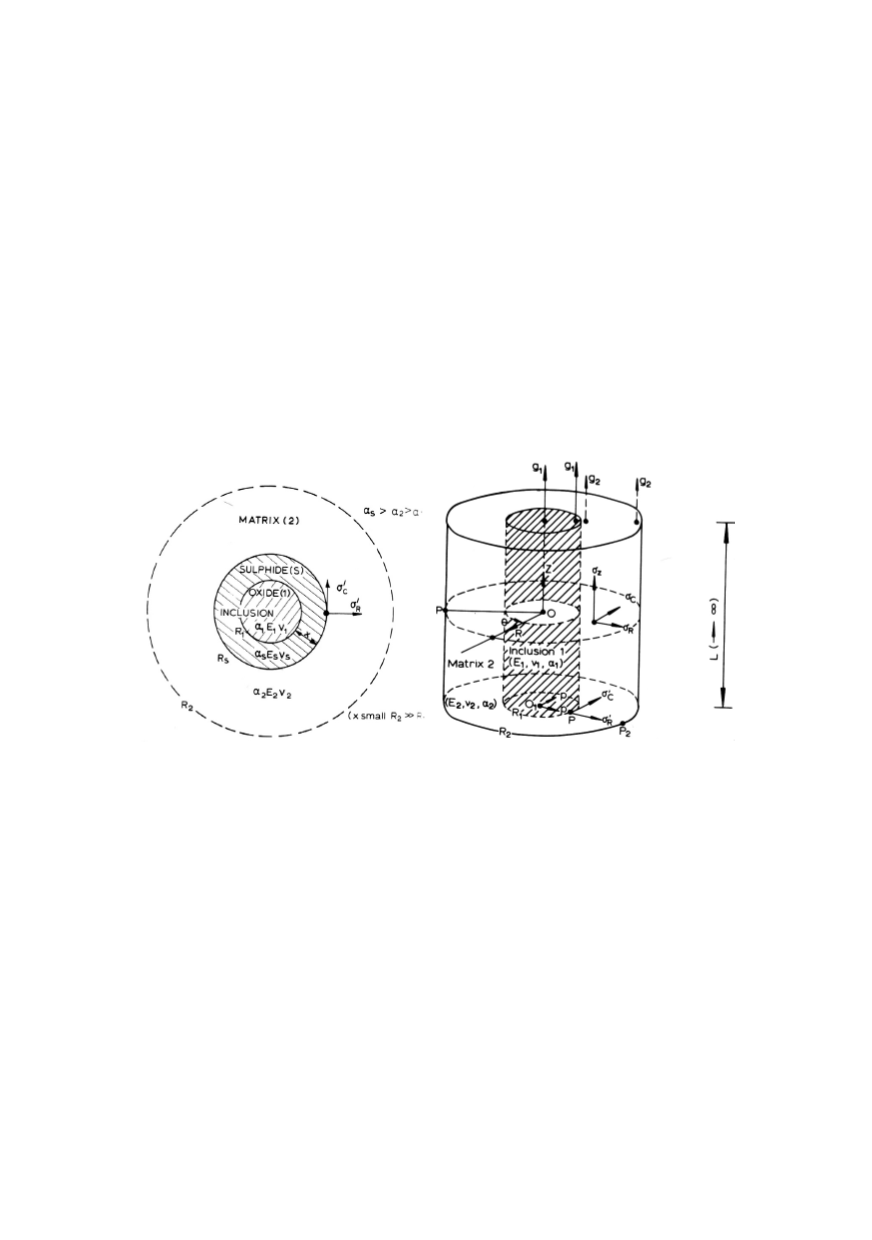
drically, cubically or else way shaped. Figure 3a shows schematically the complex oxide-
sulphide inclusion. During steel production, oxide inclusion was first to be formed,
usually because oxides have higher melting temperature in comparison to sulphides. With
further cooling, the sulphides have been formed on already present oxides. The final
chemical composition comprises the elements present in oxide and sulphide. Also, the
shape is detrimental to the surface tension between sulphide and matrix, leading to the
stress field around the inclusion, as presented in Fig. 3a. Figure 3b presents the stress
field around one cylindrical inclusion /5/.
Figure 3: The shape, stress field and chemical composition of some inclusions:
spherical duplex inclusion (left); cylindrical inclusion (right) /5/
Oxide inclusions arise from exogenous and indigenous origins. Exogenous inclusions
are formed during the melt transfer by (1) reoxidation of deoxidized and refined steel
melt when it comes into contact with air and oxidizing slag; and (2) entrainment of the
reoxidation product, slag, and refractory. Indigenous inclusions form in the ladle from the
reaction between oxygen dissolved in the melt and a deoxidizing element such as Al or Si
added to the melt. In a deoxidized and refined steel melt, indigenous inclusions are usu-
ally small in size, and hence they are less harmful, provided that they do not agglomerate
into large ones during the melt transfer. In contrast, exogenous inclusions often exist in
large sizes shortly before the melt is delivered into the mould, have limited opportunity to
be removed, and hence are more harmful /4, 5/.
The origin of sulphur in steel is predominantly from the coke. During ore reduction in
blast furnace, large amount of sulphur gets dissolved in pig iron. During next production
steps, ladle treatment of pig iron, steel production and ladle treatment of steel, one of the
main goals is to decrease the overall content of sulphur /6/. If the sulphur is remained in
steel in solid solution it can form the eutectic alloy with very low melting temperature


















